Metal-Catalyzed Azidation of Tertiary C–H Bonds Suitable for Late-Stage Functionalization
Another entry in the wonderful world of the C-H activation, this time introducing azides.
C-H activation is one of the hottest topics in organic synthesis, and lately even more, because some authors are applying these methods not for decorating scaffolds, but for the functionalization of final products. That is, the typical molecules that you have submitted to your primary assay and they look fine so far, but you would want to carry on just one step further to add a little extra. Adding an OH to increase solubility, or a methyl to see if magic happens, or whatever. We have posted recently about a paper on methylation by C-H activation.
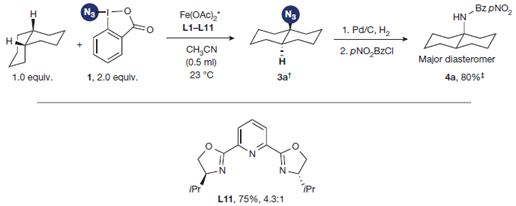
The group of Hartwig (Urbana, IL, USA) has maded another incursion, now introducing an azide. Though the work is still preliminary, it is really, really promising. Using Iron catalysis and the Zhdankin reagent (honestly, first time I heard about it… looks like the offspring of sodium azide and IBX), they can functionalize a single C-H on the molecule introducing the azide there. Just finding the conditions was probably a long work. But it payed benefits.
Besides the pure methodology examples, they tried the reaction conditions with more complex examples: some terpenes containing epoxides, aziridines and cyclopropanes, steroid intermediates and a tetrahydrogibberellic acid derivative. Yields are usually moderate, but introducing the azide in highly substituted positions, selectively, is already amazing. In other examples they play a bit with the azide, preparing amines, amides, or doing click chemistry. There are no examples with drug-like products, which is a real pity.
So the days where the final product is just an intermediate for the product with better properties are coming.
Nature 2015, 517 (29 January 2015), pp 600-604.
See: 10.1038/nature14127