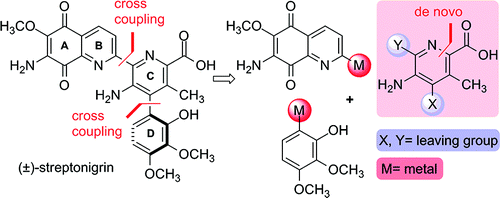
Total Synthesis of (±)-Streptonigrin: De Novo Construction of a Pentasubstituted Pyridine using Ring-Closing Metathesis
A total synthesis of Streptonigrin using a RCM to furnish the central pyridine ring.
It is the first time we include in our newsletter a total synthesis. Reasons are usually we find more useful for the med chem community those papers related with methodology, since they are the backbone of the chemistry work you will apply in the drug discovery effort. But in this case the total synthesis includes a feature that will sound familiar to many colleagues around: a pentasubstituted pyridine. Sometimes, getting the proper substitution ring is so difficult that you spend an awful amount of time playing with building blocks, reactions, additional steps and so. In this case, Donohoe et al. (University of Oxford, UK) went directly to the problem: RCM using a 2nd generation Hoveyda-Grubbs catalyst. Then oxidation, protection, bromination and it’s done! (well, at least the pyridine).
Conclusion: if you are not afraid of the challenge, success is possible. But if you block yourself with ‘too many steps’ or ‘too complicated’ statements, then you are done.
J. Am. Chem. Soc., Article ASAP. See: 10.1021/ja207835w