A Simple, Modular Synthesis of Substituted Pyridines
You have not lived until you have synthesized a highly functionalized pyridine. When the time comes, this new method will make your life easier.
Pyridines are core medicinal chemistry compounds. Their chemistry can be challenging, and if you work with them for sure you will never get bored. The synthesis of highly functionalized pyridines is really tough and there are no general methods available unless you have a very specific substitution pattern.
Now, a paper from Liebeskind’s group (Atlanta, GA, USA) addresses this problem with a synthetic method based on the coupling and cyclization of two simple substrates, a,b-unsaturated ketoxime O-pentafluorobenzoates and alkenyl boronic acids. Although they look complicated, in fact these oximes are easily prepared from widely available a,b-unsaturated ketones.
With the substrates in hand, the reaction is carried out using Cu(OAc)2 as the catalyst in DMF at 50 °C. The reaction does not need inert atmosphere, since the initial coupling is not sensitive and in fact air is needed to complete the second oxidation step, performed simply by heating at 90 °C for a few hours. The authors prepare a wide range of substituted pyridines with different substituents, with up to three rings hanging from the pyridine core. Yields are usually in the 40–90% range.
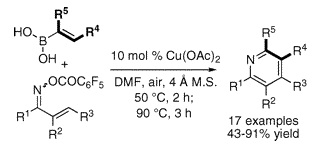
J. Am. Chem. Soc., 2008, 130 (22), pp 6918–6919. See: 10.1021/ja8013743
