A One-pot Process for the Regioselective Synthesis of 1,3,4-Trisubstituted-1H-pyrazoles
A new one-pot procedure for the regioselective preparation of trisubstituted pyrazoles.
Pyrazoles are usually grateful compounds. There are many methods for pyrazole synthesis, most of them involving condensation of 1,3-diketones with hydrazines. If you need a trisubstituted pyrazole, the problem is more complicated, and if you are looking for a regioselective method, then you usually have a problem.
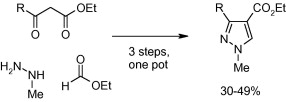
The paper of Turner and Raw (AstraZeneca, UK) tackles the synthesis of a very specific pyrazole, 1,3-dimethyl-1H-pyrazole-4-carbaldehyde. For such a simple substrate, a method good enough has not been yet reported. But since they were working on it, they have extended also the method to prepare other 1-methyl-3,4-disubstituted pyrazoles. The result is a simple, one-pot process suitable for a range of pyrazoles.
The protocol involves the reaction of methylhydrazine and methylformate in IMS under reflux. A b-ketoester is added and the solution is refluxed again; finally, NaOEt is added to the mixture. Work-up of the reaction yields products in 30–49%, not bad for three steps on one pot. The reaction is described at multigram scale. Unfortunately no aryl-b-ketoesters can be used, since the resonance with the ring diminishes the reactivity of the carbonyl against the formylated hydrazine intermediate, but the authors say that solutions are “ongoing within our laboratories”.
Tetrahedron Lett., 2009, 50 (6), pp 696–699. See: 10.1016/j.tetlet.2008.11.099