Zinc-Catalyzed Reduction of Amides: Unprecedented Selectivity and Functional Group Tolerance
A mild, selective reduction of tertiary amides catalyzed by zinc acetate.
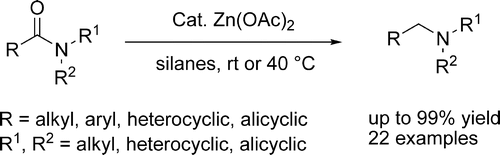
The reduction of tertiary amides is one of those reactions for which you do not have many possible options. You can use LiAlH4, BH3·SMe2 and other hydrides, usually under harsh conditions. And the chemoselectivity is not good. So what about a chemoselective, mild reducing method for amides?
The work by Beller et al. (Rostock, Germany) addresses exactly those points. They started using silanes as source of hydride, and found out that though PhSiH3, Ph2SiH2, and (EtO)2MeSiH gave excellent yields, the best silane was (EtO)3SiH. Additionally, the magic catalyst for the reaction was a Zinc one: Cu and Fe salts failed completely. Among the Zinc sources, the chosen one was Zn(OAc)2. The resulting reaction system is so nice that reactions can be carried out in a multigram scale without special precautions.
So the protocol involves mixing Zn(OAc)2 (10 mol%) and (EtO)3SiH (300 mol%) in THF for 30 minutes at r.t.. Then the amide in THF is added and the reaction stirred at r.t. for 20-30 h. The products are obtained in yields ranging from 70% to quantitative. The selectivity of the method is excellent: Ketones, nitriles, esters, nitro, double bonds (conjugated and normal flavours) stand against the system.
J. Am. Chem. Soc., 2010, 132 (6), pp 1770–1771. See: 10.1021/ja910083q