Redox-neutral organocatalytic Mitsunobu reactions
A fully catalytic Mitsunobu reaction
This article has been in our ‘to write about’ list for some time, which is a real pity because the paper is interesting indeed. This work tackles, once again, one of the most used methods to carry out the nucleophilic substitution of alcohols in one step, the Mitsunobu reaction.
This reaction was described more than 50 years ago, but it is still in use with remarkably few modifications. The reaction is triggered by the nucleophilic attack of a phosphine (most often triphenylphosphine, TPP) on the N=N double bond of an azodicarboxylate (usually diethyl azodicarboxylate, DEAD). This first step results in a betaine intermediate, which in turn reacts with the alcohol to form an alkoxyphosphonium salt. A number of pronucleophiles can then be introduced to react with this salt. In short, the result is the nucleophilic substitution of the alcohol with the nucleophile, plus triphenylphosphine oxide (TPPO, bearing the oxygen from the former hydroxy group) and diethyl hydrazine-1,2-dicarboxylate. So, although the reaction is formally a nucleophilic substitution, it proceeds in fact through a redox reaction with TPP oxidizing and DEAD reducing. The mechanism may sound a bit convoluted, but in fact is even more convoluted when you consider everything that can happen during the reaction, and that indeed happens occasionally, as such is the nature of the chemical beast. The last effort toward a fully explained Mitsunobu mechanism can be found in this paper by Andes et al.
And that is the main problem with the Mitsunobu reaction. You need not only the alcohol and your pronucleophile, but also two other reagents in stoichiometric quantities to effect the transformation, and the byproducts are, simply put, messy. If you have ever tried to get rid of TPPO you know what I mean.
There has been great interest for years in improving the reaction and pushing it to the catalytic realm, and many improvements have been made: polymer-supported reagents, more water-soluble variants of the messy stuff, attempts to convert the redox reaction into a catalytic cycle… but so far, without significant success.
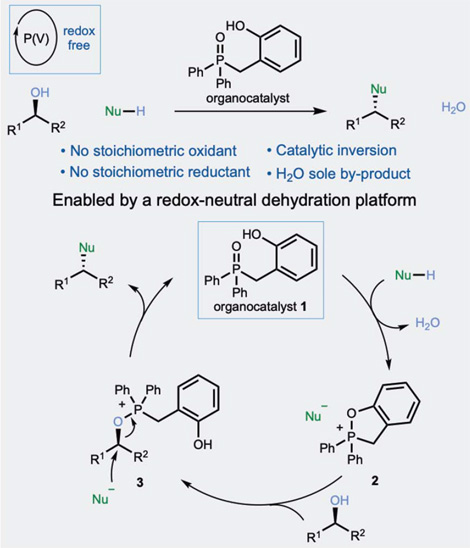
The effort here, published by researchers from the Univ. of Nottingham and GSK, replaces completely TPP and DEAD by an organocatalyst, thus transforming the reaction equation into a neat one, where the alcohol and the pronucleophile give the expected product plus water. Obviously the trick is the organocatalyst, the phosphine oxide 1. It reacts with the pronucleophile generating the cyclic phosphonium salt 2, provided that the water is removed from the medium by azeotropical distillation or so. This is critical for the reaction to proceed, because 2 will otherwise return to the phosphine oxide by hydrolysis. Then, the alcohol reacts with the cyclic salt to furnish the alkoxyphosphonium salt, which is the sought intermediate in the Mitsunobu reaction. From this point, a nucleophilic attack yields the product and regenerates the organocatalyst.
The authors carry out a nice mechanistic study with the usual C–O and C–N bond formations, along with some experiments to assess the stereoselectivity of the reaction. In summary, if this organocatalyst can replace the traditional protocol, it is a breakthrough in this field.
Science, 2019, 365, pp. 910–914. See: 10.1126/science.aax3353