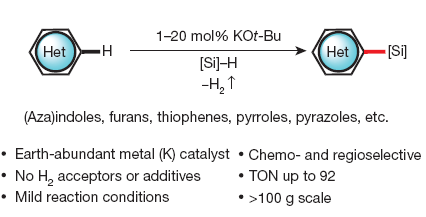
Silylation of C–H bonds in aromatic heterocycles by an Earth-abundant metal catalyst
C-H activation using a catalyst that is not based on heavy metals.
Ok, this looks like just another post about C-H activation. But believe me, it is not. First of all, it is not directly directed to activate a C-H position and couple with boronics or so. Second, it does NOT use Palladium or any other heavy metal at all. Third, it yields silanes, which are really, really useful as intermediates: further functionalization, can be used as coupling partners…
The experimental protocol is really simple: add together KOtBu (20 mol%) and the heterocycle in a vial. Add the solvent, the silane, and stir with/without heating. The protocol is carried out in a nitrogen-filled glove-box, but by first hand experience we know that this can be adapted to work using Schlenk techniques. The conditions are applied to indoles (more than 20 examples), azaindoles, benzothiophenes, 5-(2-pyridyl)thiophenes, furantes, pyrroles and others, usually with excellent yields.
Reading the paper is a pleasant experience. Well written, well detailed (for example, in the supplementary info you will find things like “Et3SiH […] filtered through a short pad of activated alumina before use“, so expect no surprises), with many additional experiments to rule out catalysis by adventitious transition metal impurities, to compare KOtBu from different vendors, to check the scalability of the reaction with 100 g, etc.
Nature 2015, 518(5 February 2015), pp 80-84.
See: 10.1038/nature14126