Selective Copper-Promoted Cross-Coupling Reaction of Anilines and Alkylboranes
This year we have worked hard on several aspects of the Copper mediated coupling of boronic acids with amines, the Chan-Lam reaction. Here we present part of the results of our work, in this case focused on the preparation of the ubiquitous phenethylamines.
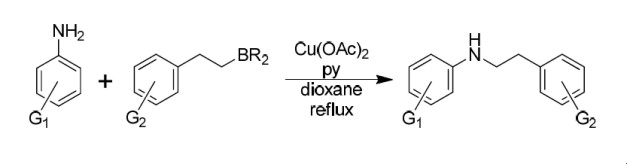
It is time for another publication in the field of Copper chemistry. Following our work on the alkylation of amines using the Chan-Lam reaction, our present research focuses on the preparation of N-arylphenetylamines, common substrates in medicinal chemistry. In a previous paper we showed that phenetylboronic acid can be used to alkylate a range of arylamines. However only phenethylboronic acid is available, raising the question what happens if we wish to generate substituted phenethylamines?
One of the answers is to use phenethylboranes as boron partners. The phenethylboranes can be prepared by hydroboration of styrenes, which are commercially available or can be easily prepared by a number of methods. For our study, we have chosen commercial styrenes. In the standard protocol, a dioxane solution of trialkylborane was prepared by the hydroboration of a styrene with BH3·THF complex. In a separate vessel, Cu(OAc)2 was added to a solution of the aniline and pyridine in dioxane. After 3-4 hours at reflux the aniline will have been consumed, leaving the monoalkylated compound in good yield.
The reaction can be run under mild reaction conditions and good functional group tolerance. Carbonyl, nitrile, halide, thioether and other groups may be present in the molecule, with yields ranging from 50 to 80%. In this case, no heteroarylamines were used. However we have much more work of a similar nature ready to be published.
This work has been accepted for publication in the Tetrahedron Letters. See: 10.1016/j.tetlet.2011.11.144.
We have another two papers ready to be written, subject to time constraints.