Titanium(IV) Isopropoxide Mediated Synthesis of Pyrimidin-4-ones
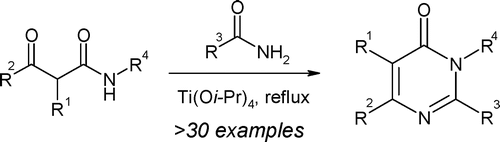
Synthesis of highly substituted pyrimidones from amides mediated by Titanium.
The pyrimidin-4-one scaffold is widespread among many pharmaceutically relevant series. There are several routes available and some of them allow the introduction of four substituents in the ring, but there are always problems involving low yields and/or low selectivity. As we have told many times before, new flexible, powerful methods for a scaffold are always welcome.
The paper by Ramanjulu (GlaxoSmithKline, Pennsylvania, USA) describes one of those powerful methods, making full use of the modern tools of the trade. The method consists in a Lewis acid mediated union and cyclization of b-ketoamides and primary amides. What makes the difference here is the Lewis acid used: it must be Titanium(IV) isopropoxide.
The reaction protocol is nice: b-ketoamide and amide (1.2 equiv) are mixed. Ti(OiPr)4 (4.0 equiv) is added and stirred at r.t. for 10 min., followed by stirring at 150 °C for 24 h. Quenching, extraction and purification yield the pyrimidin-4-ones. If you are worried about the preparation of the starting b-ketoamides, the authors provide quick microwave preparation methods from diketene (commercially available) and an amine or alternatively from a b-ketoester and an amine. The yields for the final products are only moderate, but if you think about the diversity you are introducing into a simple step, not bad at all. Many examples with heterocycles are provided, and a bromine atom can be incorporated to the cyclization product using NBS in DMF, so more diversity can be added through coupling reactions. Another advantage: The procedure can be easily scaled up. Not bad at all.
Org. Lett., 2010, 12 (10), pp 2270–2273. See: 10.1021/ol100624p