Palladium-Catalyzed Direct Arylation of Azine and Azole N-Oxides: Reaction Development, Scope and Applications in Synthesis
A demonstration of C–H activation using N-oxides of azines and azoles.
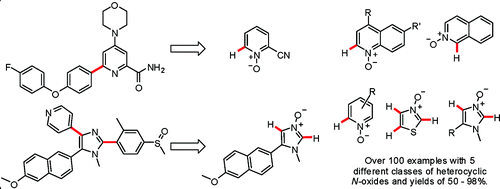
For many chemists, the N-oxides of azines and azoles are just synthetic intermediates to overcome some electronic richness problems at the rings. They are useful, yes, but only in one or two cases.
But these N-oxides of azines and azoles can be used for selective C–H activation also. A work by Fagnou et al. (Ottawa, Canada) describes the mild preparation of oxides of imidazoles, thiazoles, pyridines, quinolines, and isoquinolines and the coupling of such products with aryl bromides. Through a number of tables, the scope and selectivity of the coupling reaction on different positions of the oxides is described. The work is quite comprehensive and opens a new way to prepare a structural motif found in many natural products and drugs, an aryl group adjacent to the nitrogen atom of the ring. This method should be specially valuable when the 2-halo compound is not available. Moreover, in some cases, the activation of the ring allows some additional chemistry to be performed, functionalizing other positions.
J. Am. Chem. Soc., 2009, 131 (9), pp 3291–3306. See: 10.1021/ja808332k