Manganese-Catalyzed Late-Stage Aliphatic C?H Azidation
Another protocol to carry out a late-stage azidation using a Mn complex.
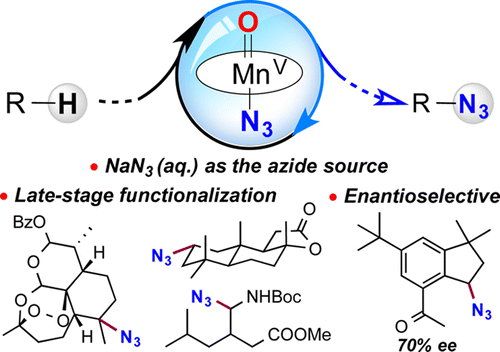
Two months ago we mentioned in this blog a paper by Hartwig about a late-stage azidation reaction of tertiary C-H bonds>. Now Groves et al. (Princeton University, NJ, USA) have published a new methodology to carry out this reaction, which is in fact a follow up of their previous work in late-stage fluorination. The protocol developed by Groves uses a Manganese catalyst (a Salen-Mn complex) and NaN3 as the azide source and is experimentally quite simple: just mix together the substrate, the Mn complex, PhIO (a stochiometric oxidant needed to oxidize the Mn(III) to Mn(V)) and stir at room temperature. The reaction can be even done under air.
The protocol is quite selective to benzylic C-H bonds, but among the substrates pool used to demonstrate the scope of the reaction you can find many different things (including some examples with heterocycles). The authors move later to more complex substrates (for example, Artemisinin and others), and they demonstrate that they can indeed functionalize some very specific positions.
And the chirality? Well, if you are using a Salen-Mn complex, using a chiral Salen-Mn complex is not much more difficult. And it works, though the ee obtained is still modest, 70%. But the authors hope to improve the method.
J. Am. Chem. Soc. 2015, 137 (16), pp 5300-5303.
See: 10.1021/jacs.5b01983