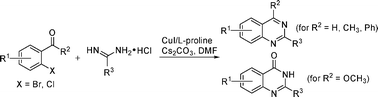Highly Efficient Copper-Catalyzed Cascade Synthesis of Quinazoline and Quinazolinone Derivatives
Amidines are useful building blocks for the synthesis of 1,3-diazaheterocycles. See how they can be used to build quinazolines.
As we have commented in previous issues of this newsletter, amidines are useful building blocks for the preparation of many 1,3-diazaheterocycles, such as imidazoles and pyrimidines. New methods are still being developed because any new broad scope procedure suitable for application in parallel is of interest for medicinal chemists.
The work of Fu and Jiang (Tsinghua University, Beijing, China) is a good example based on the rediscovered and optimized Ullmann N-arylation. The method involves the coupling of o-carbonylaryl halides (aldehydes or esters) with amidines in the presence of CuI, L-proline as the ligand, and Cs2CO3 as base in DMF at 110 °C during 16–24 h. The reaction proceeds through an initial condensation of the amidine with the carbonyl group, followed by coupling to the ring. Twenty examples are provided, with yields ranging typically from 80% to 90%. Although the method could be improved using microwaves, the reaction is easy and constitutes a good addition to the medicinal chemist toolkit.
Chem. Commun., 2008, pp. 6333–6335. See: 10.1039/b814011a