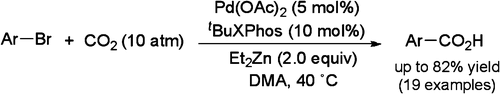Palladium-Catalyzed Direct Carboxylation of Aryl Bromides with Carbon Dioxide
A carbonylation reaction that uses CO2.
The preparation of carboxylic acids through metal-mediated coupling of an (hetero)aryl halide and CO is a well-known reaction. We have published in previous issues of this newsletter several papers related to this topic describing improved methods with expanded scope. The main limitation with these methods is the use of CO gas, which is hazardous and must be carefully controlled, although some work exist on the use of solid CO sources. Many authors have pointed out that CO2 would be a much more convenient, safer, and cheaper source for the C1 block, but success in the direct CO2 insertion in aryl halides in a catalytic manner remains elusive.
The work by Martín et al. (ICIQ, Tarragona, Spain) seems to be the first step in the right direction. The protocol described in the paper involves a pressurized reaction with CO2 (10 atm) with an aryl bromide using Pd(OAc)2 (5 mol%) as the catalyst, tBuXPhos (10 mol%), and Et2Zn (2 equiv.) in DMA at 40 °C.
The substrates are still limited to aryl bromides. The 19 examples reported include two heterocycles, although they are both thiophene derivatives.
J. Am. Chem. Soc., 2009, 131 (44), pp 15974–15975. See: 10.1021/ja905264a