Functionalized olefin cross-coupling to construct carbon-carbon bonds
A new method for the generation of quaternary centers using Fe catalysis.
Some years ago, attending an event about Pharmacology, I had a interesting chat with a med chem. In one moment of the conversation, he struck with this comment: ‘Today we can prepare everything’. In that moment I told him nothing, but the old internal wetware processor Mk. 1.0 (also known as brain), digested the comment and issued a DO NOT CONCUR message. There are still many, many structural motifs that are hard, or even impossible to prepare with the current chemical methods. Among these structural motifs are functionalized quaternary centers. And even when we have synthetic methods available, it is not straightforward.
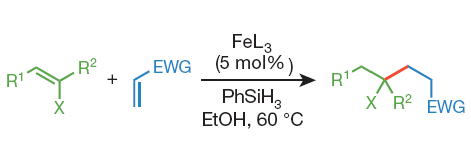
Baran (Scripps, CA, USA), has tackled the synthesis of quaternary oxygenated centers with a new catalytic method using an iron catalyst (Fe(acac)3 or Fe(dibm)3)) and PhSiH3 in EtOH. The method is easy to set up, mild and works with a surprising number of different compounds (more than 40 examples in the paper). As Baran says in the paper, ‘However, no reaction is without limitations.’. Steric hindrance hurts the reaction, but still, you can use from silyl enol ethers to vinyl boronates. Oxygen is not a problem, and even in a reaction medium heavily ‘contaminated’ with other organic compounds the protocol yields the expected product: they have run reactions in Vodka, Tequila, Wine, Whisky and others.
Talking about practical aspects, setting up the reaction is really simple (see Baran’s lab blog for a demonstration video). The Fe(acac)3 is commercial, and in the supplementary information of the paper (209 pages !!!!) more details are provided about the experiments, preparation of the Fe(dibm)3, and others.
Nature, 2014, 516 18/25 December, pp 343–348. See: 10.1038/nature14006