A Multiligand Based Pd Catalyst for C-N Cross-Coupling Reactions
Advances in the palladium catalyzed amination.
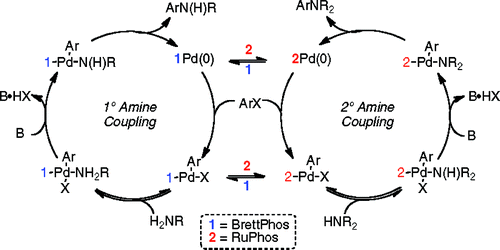
Palladium catalyzed C-N bond formation is reaching quickly a point of maturity. This recent work by Buchwald et al. (MIT, USA) shows how we have now very general conditions that can be applied to a broad pool of substrates. Fishing in their now very deep expertise pond they thought of combining some of the best catalyst known to date. So they carried out experiments using mixtures of RuPhos and a precatalyst based on the BrettPhos ligand. With a proper use of base, they can couple not only primary, but also secondary amines using this system. This is quite interesting, because it is known that BrettPhos does not work for the coupling of secondary amines, and RuPhos has a similar behaviour in the coupling of primary amines. The mixture of both systems not only does not interfere, but takes the best of both systems to provide a general catalytic system. The authors tried the new mixture on a pool of primary, secondary and aromatic amines with excellent results, and also with benzamide (a common ammonia surrogate). To top it off, the system works nicely on aromatic chlorides, thus expanding the scope of the method. As the authors state “We hope that the use of multiligand systems will facilitate the development of more comprehensive catalysts and, ideally, lead to the discovery of a truly “universal” system.”
J. Am. Chem. Soc., 2010, 132 (45), pp 15914–15917. See: 10.1021/ja108074t