Organic Photocatalysis for the Radical Couplings of Boronic Acid Derivatives in Batch and Flow
Eight years ago, we included in our newsletter an entry about a photochemical oxidation in flow, because we thought it was part of an increasing trend in synthesis. It seems our insight was right, because modern photochemistry methods have made a great impact in the pool of synthetic options available to chemists. For those of us who spent months during our PhD performing radical reactions with stoichiometric reagents, visible-light photoredox catalysis is like magic and a great improvement from the old days. One of the best starting points for newcomers to this field is the excellent site of McMillan’s group, the Merck Center for Catalysis at Princeton University. In this site, you will find tips and tricks on available reactions, LED lamps, reactors, and so on.
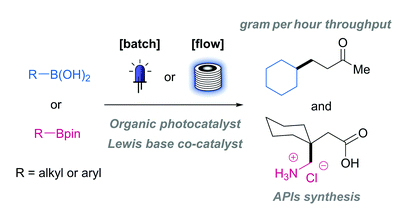
One of the limitations of the current reactions is the effective oxidation of the redox-active complexes, which relies on costly iridium-based catalysts. This paper by Steven Ley et al. (University of Cambridge, UK) aims to overcome this limitation by looking for a suitable organic replacement to the previously used iridium photosensitizers. This is specially interesting for the synthesis of pharmaceuticals and others. In the ICH Q3D Guideline for Elemental Impurities, Ir is grouped with Os, Ru, and Rh in terms of toxicity, so avoiding residual traces of this metal would expand enormously the scope of industrial photoredox reactions. The model reaction selected is the radical conjugate addition reaction of a boronic ester or boronic acid with methyl vinyl ketone (MVK) as the radical acceptor. Using blue LEDs (14 W at 450 nM) and a Lewis base (DMAP or quinuclidin-3-ol), they discovered that Rose Bengal works fine as a replacement, but even better are two recently developed dyes, 4CzlPN and Mes-Acr-4.
After some successful results using NMR tubes (reaction completion within 70 min), they attempted several reactions in batch to prepare APIs of the g-amino butyric acid (GABA) family (baclofen, phenibut, pregabalin, and gabapentin). Again, good results were obtained by telescoping the deprotection, hydrolysis, and decarboxylation using aqueous HCl in one single step.
Flow chemistry is well suited to reactions with short reaction times that perform well at the microscale. If it is true for microwave reactions, it is true for photochemical reactions, but in this case is not a question of heat but light. The Photosyn module of the Flowsyn reactor (Uniqsis Ltd.) was applied to the problem and the photoredox reaction was optimized successfully, thus paving the way for potential scale-up in larger flow photoreactors.
Chem. Commun., 2018, 54, pp 5606–5609. See: 10.1039/c8cc02169d