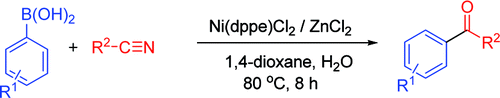Direct Synthesis of Arylketones by Nickel-Catalyzed Addition of Arylboronic Acids to Nitriles
Ketones from boronic acids and nitriles
One of the first contracts we carried out in GalChimia was the preparation of a ketone present as impurity in a process for an API. The synthesis relied in the addition of an organomagnesium compound to a nitrile. The nitrile was commercially available, but the organomagnesium compound had to be prepared from the corresponding bromide, magnesium turnings and iodine or 1,2-dibromethane as promotor.
A modern version of this reaction can be seen in this paper by Cheng et al. (National Tsing Hua UniVersity, Taiwan). Their studies follow the trail of other metal mediated protocols for the addition of boronic acids to nitriles, but in this case using a first-row transition metal: Nickel. The use of different bidentante Ni complexes and a Lewis acid allows the succesful addition of different boronic acids to the nitriles. In a typical protocol, a sealed tube containing Ni(dppe)Cl2 (10 mol%), ZnCl2 (150 mol%) and boronic acid (200 mol%) is purged and then the nitrile, H2O (100 mol%) and 1,4-dioxane were sequentially added to the system and the reaction heated at 80 °C for 8 h.
Two striking points about the protocol are the introduction of water to enhance the yield, something not expected when using ZnCl2, and the absence of base; no base is necessary for the transmetalation of arylboronic acid to the nickel center. The protocol is efficient and gives good yields. Since the reaction system uses 1,4-dioxane as solvent, microwave heating should not be specially effective here, but the presence of water and the zinc salt could change that, so it is worth giving it a try. No examples of heterocycles are given, so there is room for more work.
Org. Lett., 2010, 12 (8), pp 1736–1739. See: 10.1021/ol1003252