A Powerful Palladium-Catalyzed Multicomponent Process for the Preparation of Oxazolines and Benzoxazoles
Synthesis of oxazolines and benzoxazoles through a new MCR.
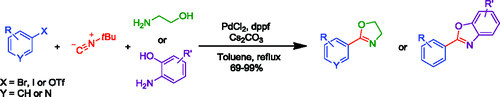
A recent paper by Lang et al (University of Strathclyde, UK) describes a new multicomponent reaction for the preparation of oxazolines and benzoxazoles. Being a multicomponent method, you just mix everything, stir and that is, you have your product. The reaction works in fact in cascade, starting with the oxidative addition of palladium to a haloaromatic compound (Cl, Br or I work), followed by the 1,1-insertion of t-butylisonitrile and then reductive elimination with the hydroxyamino component providing the oxazol ring. The authors publish 10 examples of oxazolines (including one example using 3-bromopyridine) and 5 examples of benzoxazoles. Yields are usually excellent. One criticism: most of the halobencenes used have only one substituent in para, so a true estimation of more hindered substrates is a bit difficult. And an idea: using aminothioethanol would yield thioxazolines?
Org. Lett., Article ASAP. See: 10.1021/ol202725y