Oxygen and Base-Free Oxidative Heck Reactions of Arylboronic Acids with Olefins
The Heck reaction has been around for more than 25 years and comes in two flavors: oxidative and non-oxidative.
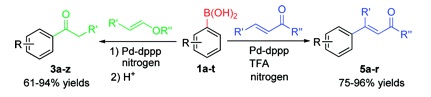
The classical Heck reaction involves the palladium-catalyzed coupling of alkenes with halobenzenes. Many variations have been devised from that starting point, including different reaction conditions, substrates and others. Although the original flavor requires the use of Pd(0), the coupling of organoboronic acids and olefins, known as the oxidative Heck reaction, involves the use of Pd(II). The methods developed require the presence of an oxidant to reenter Pd(0) in the catalytic cycle. Some oxidants, which are added in stoichiometric amounts, generate waste, suppressing the advantage of using boronic acids. The research group of Xiao (Liverpool, UK) has published a paper reporting new conditions for the reaction. The method relies on the use of acetone as the solvent to trap the X–Pd–H intermediate without forming Pd(0). The reaction yields excellent results for aryl ketones using n-butyl vinyl ether and boronic acids. When an electron-deficient alkene is used, sometimes an acid additive is introduced: TFA makes the trick. So the oxidative Heck reaction has been expanded to require no base and no oxygen. Believe me, this is a great step forward from the days of using thallium to prepare alkyl aryl ketones…
J. Am. Chem. Soc., 2008, 130 (8), pp 2424–2425. See: 10.1021/ja0782955