Advances in Carbonylation
Carbonylation has changed a lot from its pioneering reactions at high pressure and is becoming an everyday reaction.
Common methods for the introduction of a carboxy moiety into a substrate involve usually metallation and trapping with a suitable electrophile. Carbonylation has been around as an alternative for some time, but presents some drawbacks that have discouraged most chemists: high pressures, use of a toxic gas, and harsh conditions, among others. I remember seeing during my PhD some people doing carbonylations and they gave me the feeling of handling a nuclear reactor. In the last years, some work has been done to expand the scope of this reaction and so, it has become a common tool of the medicinal chemist weaponry.
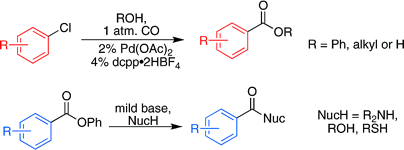
We present here two works by Buchwald (Cambridge, Mass, USA). The first one is an effort to apply the reaction to aryl chlorides, cheaper substrates. This is a trend in modern organometallic chemistry, which has long ago overcome the limits of the Br/I/OTf triad. Additionally, the group of Buchwald has centered the work in the preparation of esters, but only as acyl transfer reagents for the in-situ preparation of amides. However, having succeeded in the preparation ofphenyl esters and their transformation into other esters and amides, they have expanded the protocol for the preparation of the products in a one-pot procedure. As an example, an aryl chloride is reacted with CO at room pressure, using Pd(OAc)2, dcpp·2HBF4, K2CO3, and molecular sieves in DMF or DMSO. Different alcohols can be introduced (MeOH, EtOH, PhOH…). Substrates include aryl and heteroaryl chlorides with yields ranging from 60% to 85%. This is a worthy alternative to introduce an ester from some common scaffolds overcoming old methods.
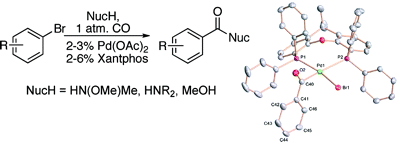
The second paper is quite similar, involving the carbonylation of aryl halides with CO at atmospheric pressure, but there are two differences. First, the substrates are aryl bromides. Second, the reaction allows the preparation of not only esters, but amides and Weinreb amides in a one-pot procedure. The standard protocol involves heating the aryl bromide with the nucleophile (MeNHOMe·HCl, an amine or MeOH) using CO at room pressure, Pd(OAc)2, Xantphos and a base (K2CO3, K3PO4, or Et3N) in toluene. The range of substrates includes aryls and heteroaryls and the yields are excellent. The preparation of these compounds has never been so easy.
Carbonylation of Aryl Chlorides with Oxygen Nucleophiles at Atmospheric Pressure. Preparation of Phenyl Esters as Acyl Transfer Agents and the Direct Preparation of Alkyl Esters and Carboxylic Acids
J. Org. Chem., ASAP Article. See: 10.1021/jo800907e
Palladium-Catalyzed Carbonylation Reactions of Aryl Bromides at Atmospheric Pressure: A General System Based on Xantphos
J. Org. Chem., ASAP Article. See: 10.1021/jo801279r