Water works: an efficient palladium-catalyzed cross-coupling reaction between boronic acids and bromoacetate with aminophosphine ligand
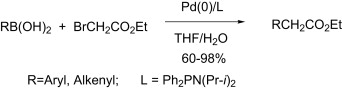
Preparation of phenylacetic acids in water through a palladium coupling.
I must admit that I have an issue with phenylacetic acids. My PhD was centered on synthetic applications of these products, and synthesizing these things was not easy 15 years ago. The usual reaction involved taking a benzaldehyde, reducing it to a benzylic alcohol, changing the hydroxy group into a halide, and then introducing a nitrile using sodium or potassium cyanide. Then you hydrolised the nitrile and presto!, you had the phenylacetic acid. Just writing it is tedious. Obviously, many variations are allowed: reduction of a benzoic acid, benzylic bromination of a toluene, etc. In some special ocasions you could try the usual homologation of a benzoic acid using diazomethane (that scared everybody around, by some reason they expected me to blow up the whole department and were always very disappointed) or, more safe but tedious, a Wittig reaction with the methoxymethylidenphosphorane followed by a hydrolysis.
I am very happy to say that phenylacetic acids are more accesible today, specially seeing papers like this by Zhang et al. (Shanghai, China). They have developed a nice protocol that makes use of boronic acids as starting materials and ethyl bromoacetate as the two-carbon building block. In a typical reaction, the boronic acid, K2CO3 (200 mol%), Pd2(dba)3 (1.6 mol%), ligand (5 mol%), THF, water and ethyl bromoacetate are charged sequentially into a degassed Schlenk tube (or similar). The system is degassed again and heated at 65 °C for 8 h. Though it can look tedious, in fact this protocol is quite common and similar to many other coupling protocols. Results reported are excellent for a pool of 16 different substrates, with the usual yields around 60%. No heterocyclic substrates are included. The only drawback of the method is that the ligand, an aminophosphine, is not commercial and must prepared.
Tetrahedron, 2010, 66, pp 8238-8241. See: 10.1016/j.tet.2010.08.047