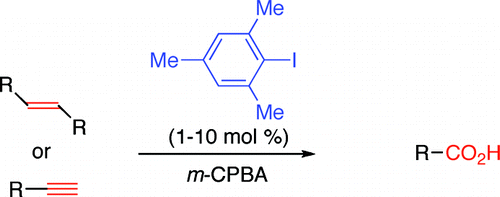
Iodomesitylene-Catalyzed Oxidative Cleavage of Carbon–Carbon Double and Triple Bonds Using m-Chloroperbenzoic Acid as Terminal Oxidant
Oxidative-cleavage of double and triple bonds without hazardous ozone or metallic oxidants.
The classical method for breaking up doble carbon–carbon bonds is ozonolysis. However, this method is less than perfect, requiring specialized equipment, a hazardous reagent, and a complicated protocol. Other methods for this oxidative cleavage are available, but involve using powerful oxidants in stochiometric quantities.
The method published by Ochiai et al. (Tokushima, Japan) relies on the formation of aryl-λ3-iodanes, which are environmentally friendly, using m-chloroperbenzoic acid (mcpba) as the oxidant and iodomesitylene as the catalyst. Two protocols are described using CH2Cl2-HFIP-H2O or CH3CN2O as the solvent, both with HBF4 as the acid. Specially interesting is the application of the method to arylalkynes, although only two examples are provided. The method could be used as an alternative to carbonylation.
J. Am. Chem. Soc., 2009, 131 (4), pp. 1382–1383. See: 10.1021/ja808829t