General and User-Friendly Protocol for the Synthesis of Functionalized Aryl- and Heteroaryl-cyclopropanes by Negishi Cross-coupling Reactions
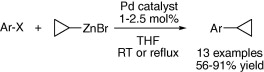
Introduction of cyclopropyl rings by Negishi coupling.
The importance of the cyclopropyl ring in SAR studies is well known to medicinal chemists. In particular, the higher metabolic stability of the cyclopropyl group compared to aliphatic residues toward microsomal oxidation makes then very interesting substrates when other groups must be discarded. Many attempts have been made in the last years to expand the methods used for the introduction of this structural motif, from the original cyclopropanation reactions to the modern organometallic couplings. Most of them have been tried and there is a broad range of options available, but some things can still be improved.
The work by Leitner (BASF) focuses exactly on those weak points, more specifically the synthesis of a cyclopropyl reagent in high yield and a few steps, with a shelf life of several months, broad substrate scope, and tolerance to sensitive functional groups. Add to the mixture that it must be cheap and you need hundreds of kilos, and you have a real challenge. In a few words, they have succeeded in preparing cyclopropylzinc bromide using a Rieke zinc methodology. This reagent can be coupled to a broad scope of substrates using a catalytic system composed of Pd(Ph3P)4 and PEPPSI in THF at r.t. or reflux to give the final compounds in excellent yield. Thirteen examples, including several heterocycles, are described.
Tetrahedron Lett., 2009, 50 (31), pp. 4475–4477 See: 10.1016/j.tetlet.2009.05.054