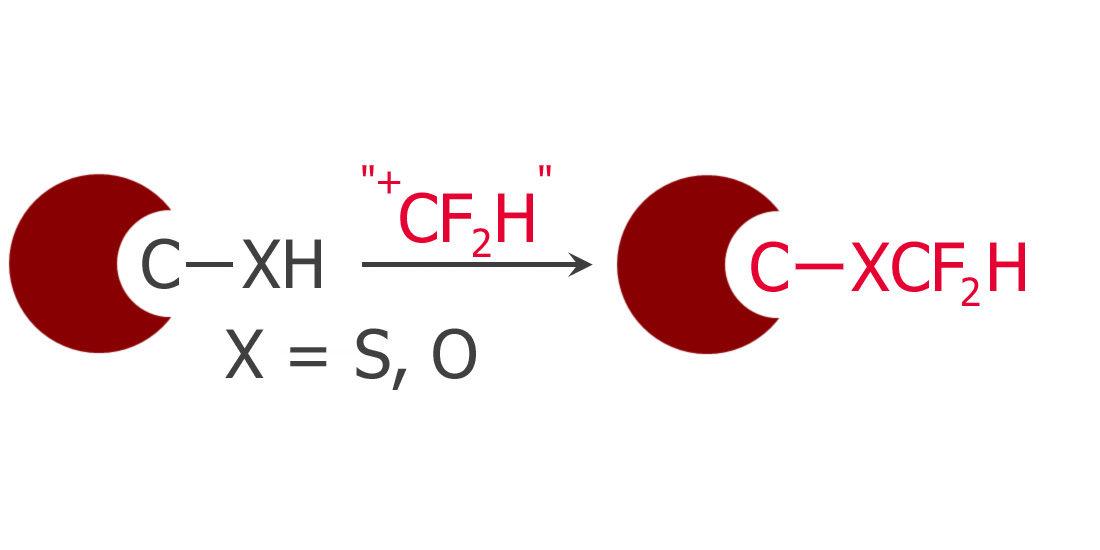
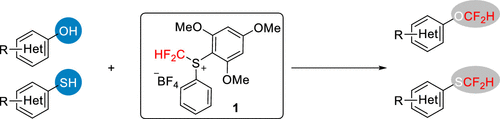
Difluoromethylation of Phenols and Thiophenols with S-(Difluoromethyl)sulfonium Salt: Reaction, Scope and Mechanistic Study
A new reagent for the introduction of the CF2H moiety
It is funny how these things happen. You can spend months working on a project where you need to introduce a very specific group (a CF3, a geminal difluoride, a nitrile…), trying different options available in the literature without any luck. And one day, once the project is closed and you have given up on that transformation… a new paper is published with a method that can solve your problem.
There is a new player on the field of difluoromethylation. And it is a difficult field indeed. We have moved from the use of CFCs (or similar) as source of the CF2 group to less harmful reagents, though more limited in scope. Things like FSO2CF2COOH, PhCOCF2Cl, HCF2OTf and others… But it is not easy, and not long ago Hu introduced a better protocol using the commercially available TMSCF2Br. No luck with that also, thanks.
This new reagent by Liu et al. is not commercially available. It must be prepared in three steps from thiophenol, according to the supplementary info in this paper. But once available, it seems it has a broad scope. There are 38 examples of phenols and thiophenols, including 10 examples with heterocycles. The reagent is compatible with a number of functionalities both in para- and ortho-positions. And more interestingly, it exhibits chemoselectivity between aromatic and aliphatic alcohols, and also between oxygen and sulphur nucleophiles, which the authors demonstrate with a remarkable series of experiments.
In short, this should be a worthwhile addition to the organic chemist’s toolbox for CF2H introduction.
J. Org. Chem., 2019, 84(24), pp. 15948–15957. See: 10.1021/acs.joc.9b02424