We have known for a long time that medicinal chemists tend to use only a few reactions. They are slow to incorporate new reactions to the toolbox, but once they do, those new tools are used extensively, maybe even too often. As Abraham Maslow said in 1963, «I suppose it is tempting, if the only tool you have is a hammer, to treat everything as if it were a nail».
Some years ago, a study showed the prevalence of certain reactions in medicinal chemistry. Amide bond formation was at the top, but the popularity of organometallic couplings was also clear, particularly the Suzuki and Buchwald–Hartwig reactions. It seemed thus that the new hammer were C–C and C–N bond formations. However, the Suzuki reaction is very good only for C(sp2)–C(sp2) bond formation and, even with the use of trifluoroborates, it presents certain limitations. Moreover, the products you obtain by attaching a string of biaryls are not always very drug-like. Therefore, we do need more specific hammers, because forming C(sp2)–C(sp3) bonds is a good way to make a compound more drug-like.
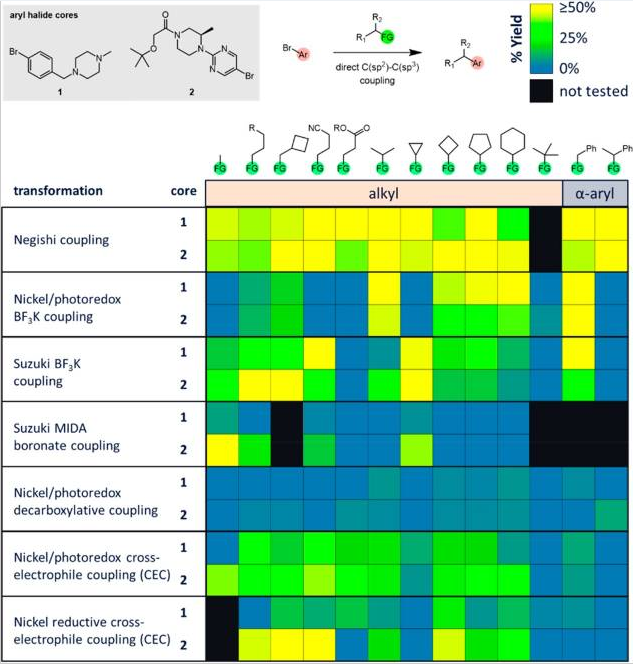
So, the question is, what tools do we have that can be used for this task? Well, according to a recent study by Dombrowski et al. (Abbvie, Northwestern University, and Florida A&M), many more than those usually employed. They ran tests comparing the ability of seven coupling methods to directly install a diverse set of alkyl groups on “drug-like” aryl structures via parallel library synthesis. The options selected were three palladium-catalyzed reactions using alkyltrifluoroborate salts and alkyl MIDA boronates (Suzuki coupling), alkyl zinc reagents (Negishi coupling); a nickel catalyzed reaction with alkyl bromides; and three nickel/photoredox dual catalyzed couplings using alkyl trifluoroborate salts, alkyl carboxylic acids and alkyl bromides.
Just to give you an idea of the typical «drug-like» fragments applied in the study, the authors included groups such as cyclopropyl, cyclobutylmethyl, aminomethyl (protected), etc. As you can see, nothing especially exotic and, in fact, the typical fragments one would sometimes struggle to introduce. The results are shown in a colored chart with the transformation and structural motif in the axes and they are somewhat striking. In summary, when handling typical alkyl fragments, the Negishi approach is by far the best option, with the alkyltrifluoroborate salts (both in the common palladium coupling and the nickel/photoredox coupling) in second place. The remaining methods are nothing special, while the MIDA boronate coupling and nickel/photoredox decarboxylative coupling look like bad ideas. Things are a bit different when they evaluated the insertion of alkyl groups containing polar functionalities (e.g., aminomethyl). The nickel/photoredox coupling of alkyltrifluoroborate salts is the clear winner, because the Negishi coupling simply cannot be used in half of the reactions (although when you can, it works beautifully). The palladium coupling of alkyltrifluoroborate salts works poorly and, once again, the nickel/photoredox decarboxylative coupling seems a bad approach.
Overall, the methods show good complementarity and as the authors state:
“The availability of the desired building block(s) should be considered when choosing a method. For a methyl group, the best methods are Negishi, Suzuki BF3K coupling, Suzuki MIDA coupling, or nickel/photoredox CEC. For primary alkyl groups, Negishi, Suzuki BF3K coupling, or nickel/photoredox CEC is the most reliable. For secondary alkyl groups, Negishi, nickel/photoredox BF3K coupling, nickel/photoredox CEC, and nickel reductive CEC give the best results. For benzylic groups, the Negishi, nickel/photoredox BF3K coupling, or Suzuki BF3K couplings are best. α-Oxy alkyl groups couple the best with nickel/photoredox BF3K or nickel/photoredox decarboxylative couplings. For α-amino alkyl groups, nickel/photoredox BF3K, nickel/photoredox decarboxylative, or Suzuki BF3K couplings are recommended. Secondary benzylic and tert-butyl groups are challenging to couple using these methods.”
Expanding the Medicinal Chemist Toolbox: Comparing Seven C(sp2)–C(sp3) Cross-Coupling Methods by Library Synthesis ACS Med Chem Lett. 2020, 11(4), 597–604. See: 10.1021/acsmedchemlett.0c00093