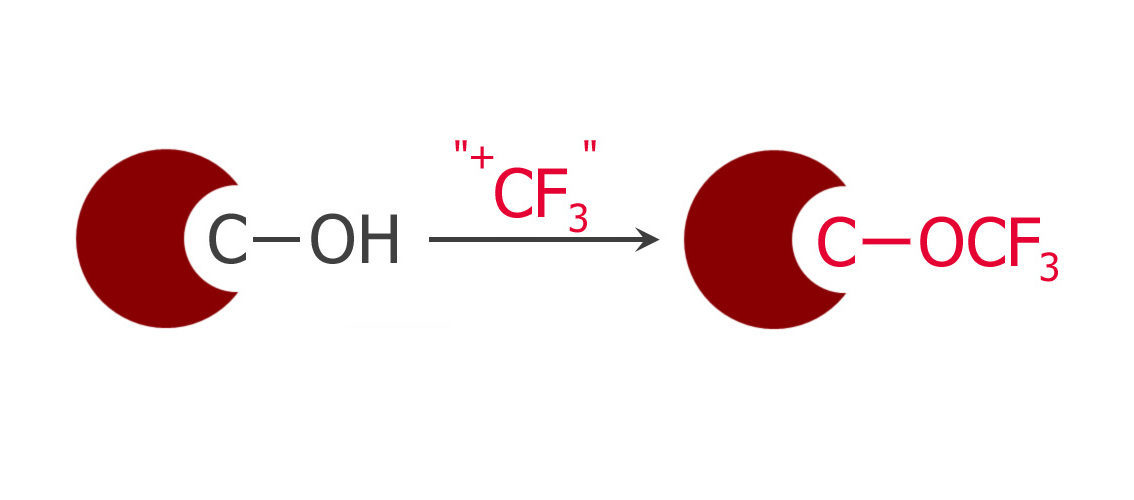
Sequential Xanthalation and O-Trifluoromethylation of Phenols: A Procedure for the Synthesis of Aryl-trifluoromethyl Ethers
Two-step method for the preparation of aryl-OCF3 compounds
This paper came to our attention thanks to one of our Project Managers, always watching for new useful reactions. A quick look at the paper’s graphical abstract is usually quite informative. It uses a xanthate (dithiocarbonate) as the intermediate, which is interesting because some time ago we reviewed here another paper for a method transforming dithioesters into trifluoromethyl groups. So this paper looked like a natural extension of the previous method, or maybe not?
After reading the paper, yes and no. The formation of the xanthate is not trivial, far more complicated than preparing a dithioester. After trying a series of xanthalating agents, they finally selected imidazolium and benzimidazolium reagents. This way the preparation of the xanthate is quick and efficient, also saving one from having to use smelly CS2.
The reagent for the next step is XtalFluor-E, a commercially available, user-friendly electrophilic fluoride source, with trichloroisocyanuric acid (TCCA) as activant. Other potential fluoride sources were tested: PyFluor, HF/KF, HF/Pyr, DeoxoFluor, DAST, XtalFluor-M, and XtalFluor-E without TCCA.
The scope of the reaction is also good. A total of 25 examples, including arenes and heteroarenes (pyridines, quinolines, and pyrazoles). Since the method is, as advertised, metal-free, it is compatible with the presence of halogens in the structure. So that’s it, another trifluoromethylation method in the bag of tricks.
J. Org. Chem., 2019, 84(24), pp. 15767–15776. See: 10.1021/acs.joc.9b02717