Palladium-Catalyzed Synthesis of Diarylmethanes: Exploitation of Carbanionic Leaving Groups
An interesting way for the introduction of a methylene bridge between aryl rings.
Welcome to the first decade of the 21st Century. The synthetic reactions inventory has grown to include an amazing number of organometallic coupling reactions. They are powerful, they are reliable, and they are flexible. They are so common that most medicinal chemistry routes include at least one. We can create almost any type of bond between aryl bonds using coupling reactions, both directly and through a bridge.
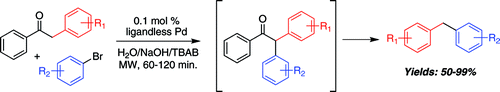
However, although the formation of Aryl-CH2-Aryl bonds is possible the reaction is still far from efficient. Most methods involve the palladium-catalyzed cross-coupling of boron species with benzyl halides. Benzyl halides are readily available, but the boron species must be prepared, usually from an aryl halide. This paper by Leadbeater (University of Connecticut, USA) shows how to overcome this limitation using directly an aryl halide, and the coupling partner is not a benzyl halide, but an α-arylketone. In a typical reaction, an aryl bromide, deoxybenzoin, TBAB, NaOH(aq), and PdCl2 are heated under microwave irradiation at 130 °C for 30 min followed by 160 °C for another 30 min. The intermediate α-arylation product losses acetophenone to yield the diarylmethane in very good yield (50–99%). No heterocycles are used as substrates. Substituted deoxybenzoins can be used as coupling partners to introduce, for example, 4-methylbenzyl and 4-chlorobenzyl groups.
Org. Lett., 2009, 11 (12), pp 2575–2578. See: 10.1021/ol900874z