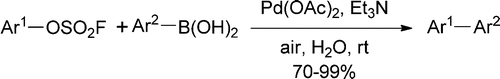Palladium-Catalyzed, Ligand-Free Suzuki Reaction in Water Using Aryl Fluorosulfates
Not only we can couple the most exotic moieties, we can do it bareback!
This paper is the fruit of a collaboration between one of the big figures in catalysis, K. Barry Sharpless (The Scripps Research Institute, CA, USA) and two chemistry groups in Shanghai, China. They have worked in developing a new class of partners for the Suzuki coupling, aryl fluorosulfates, which were until now prepared from phenols and fluorosulfonic acid. However, in 2014 the Sharpless group reported a new safer, cheaper method to prepare these compounds using Sulfuryl Fluoride (SO2F2, or abbreviated SF) as key reagent, with a protocol which resembles the introduction of any other RSO2 moiety.
With an easy access to fluorosulfates (and now commercially available), they concentrate their efforts in the coupling of the substrates. After some tweaking, the conditions selected make use of Pd(OAc)2 and Et3N in water at r.t.. No ligand, no organic solvent, no heating. The results are usually excelent, though the selection of fluorosulfates is obviously limited. Unfortunately, no many examples with ortho- substituents (in the fluorosulfate or the boronic acid), and no heterocycles. But in any case, a new group to be considered if you are thinking in a Suzuki coupling.
Org. Lett. 2015, 17(8), pp 1942–1945.
See: 10.1021/acs.orglett.5b00654