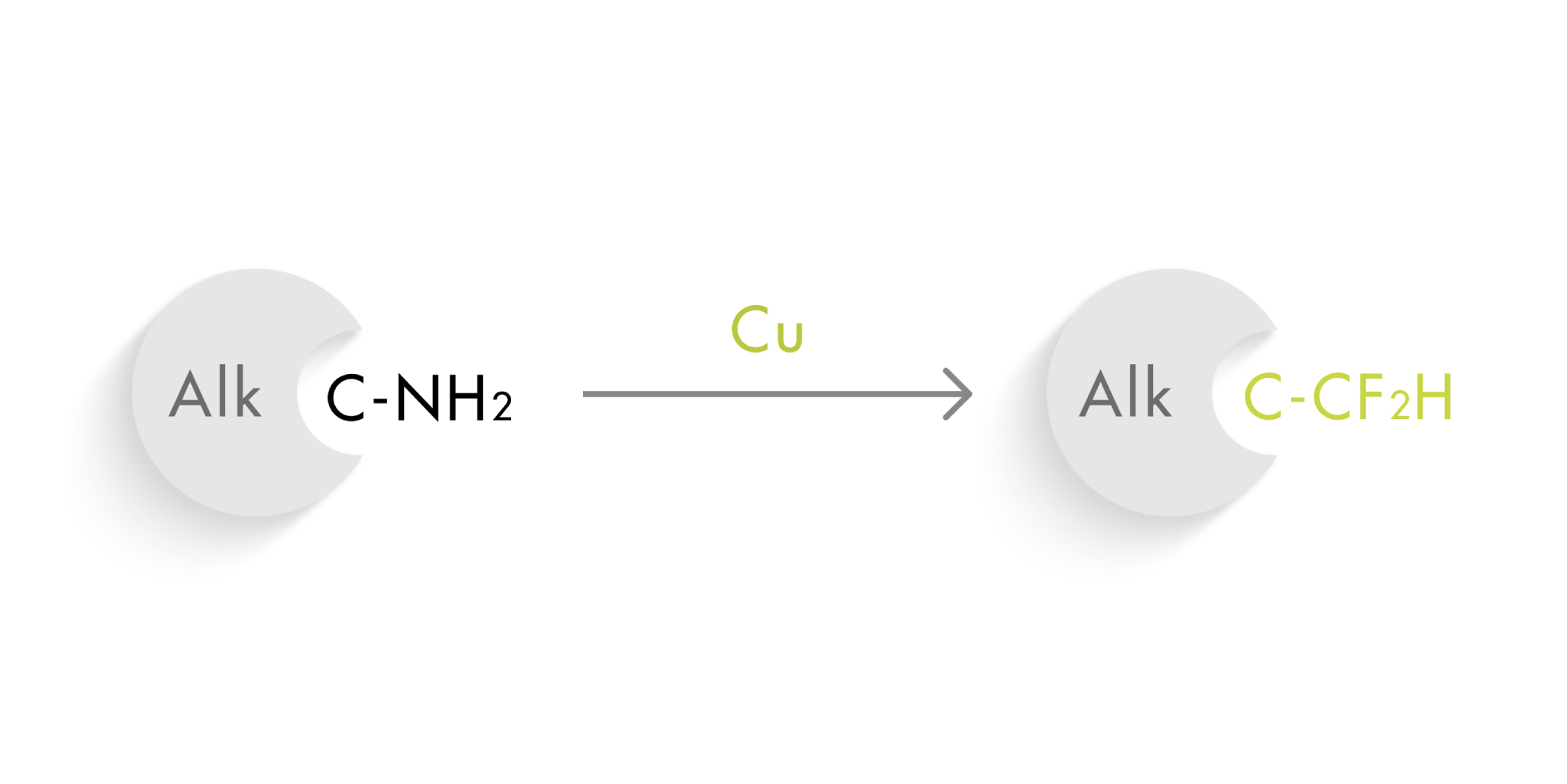
The term ‘isostere’ was coined by Irving Langmuir in 1919. One century later the concept still applies and is used daily by medicinal chemists. Some of the isosteres in use today are somewhat surprising, since the classical isostere relies in replacing one atom by another. For example, the nitrogen in –NH2 can be replaced by an oxygen, becoming an –OH. They have similar properties in terms of a hydrogen bond donor, but the CF2H group is not the typical group you would consider a hydrogen bond donor. And not only that: it is a lipophilic hydrogen bond donor.
So obviously, this moiety has great interest for medicinal chemists looking to replace, let’s say, an aliphatic amino group with another hydrogen bond donor. However, the usual difluoromethylation methods usually call for the introduction of this group in early steps of the synthetic route and are not suitable for the direct replacement of a NH2 group with CF2H.
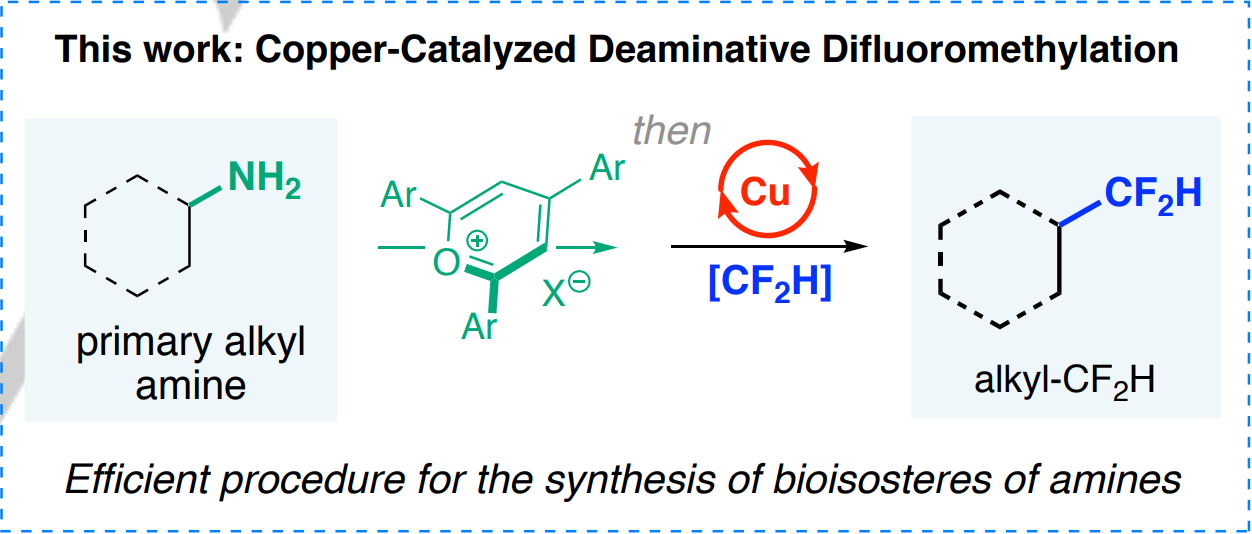
Wei et al. (Miami University, OH, USA) have come up with a new method than can replace aliphatic amino groups with difluoromethyl groups in a rather straightforward manner. The method requires the initial conversion of the alkylamino into an alkyl pyridinium salt, which is in turn treated with a copper catalyst (Cu(CH3CN)4BF4) and the Vicic–Mikami reagent as the CF2H source.
Pros and cons of the protocol: it works at room temperature with many different alkylamino moieties in carbocycles and heterocycles (about 40 examples), and it can be used as an LSF method (demonstrated by the conversion of four amino-containing drugs). But it cannot handle (hetero)aromatic amino groups (who needs anilines as drugs anyway?), requires the ad hoc synthesis of the Vicic–Mikami reagent (a Zn organometallic complex), and you will have to prepare also the reagent to convert the alkylamino into the corresponding alkyl pyridinium salt. Finally, since the deamination yields a radical that recombines with the [CuII-CF2H] species, the stereochemistry of the center bearing the amino moiety is lost in the process, affording racemic mixtures in all cases.
Copper‐Catalyzed Deaminative Difluoromethylation. Angew. Chem. Int. Ed., 2020, 59(38), pp. 16398–16403. See: 10.1002/anie.202006048