Synthesis of Cyclic Enones via Direct Palladium-Catalyzed Aerobic Dehydrogenation of Ketones
Synthesis of cyclic enones with a Palladium catalyst.
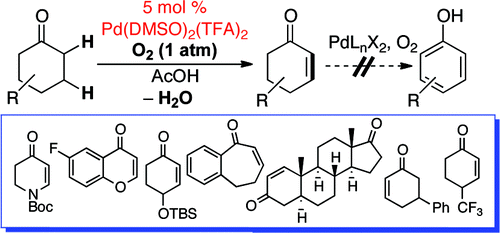
Stahl (University of Wisconsin-Madison, USA) has just published a new method for the direct formation of cyclic enones using the corresponding ketones as starting materials. No bromine and elimination, but a new Palladium catalyst: Pd(DMSO)2(TFA)2 and oxygen as oxidant. The method gives good results with many different substrates, including 4-piperidones and do not mess with labile groups like OTBS, N-Boc and others. 16 examples are reported, with some surprising selectivities when regioisomers are posible. The system can be really useful for chromone and flavone scaffolds.
J. Am. Chem. Soc., 2011, 133 (37), pp 14566–14569. See: 10.1021/ja206575j