C–H Bonds as Ubiquitous Functionalities: A General Approach to Complex Arylated Pyrazoles via Sequential Regioselective C-Arylation and N-Alkylation Enabled by SEM-Group Transposition
A general approach to highly functionalized pyrazoles using C–H bonds.
Pyrazoles are common scaffolds in medicinal chemistry. As we said in our previous issue, most preparation methods for these compounds involve condensations of hydrazines with dicarbonyl compounds or equivalent synthons, methods which lack sometimes suitable selectivity, but that can be used to prepare highly functionalized pyrazoles with every position bearing a substituent.
The group of Sames (Columbia University, USA) has published several papers on this issue, but this article is specially interesting for several reasons. First, as we said before, it focuses on pyrazoles, which are specially interesting. Second, they have developed a new methodology. And third, they have mapped the reactivity of a substituted arylpyrazole scaffold, providing a rationale approach for other researchers applying the methodology.
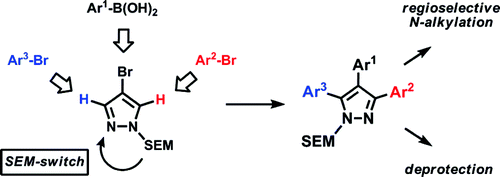
The method relies on the use of a 2-(trimethylsilyl)ethoxymethyl (SEM)-protected 4-arylpyrazole, prepared by a sequence of bromination, Suzuki arylation, and protection. The reactivity of this scaffold in the reaction with ArBr (1.5 equiv.), Pd(OAc)2 (5 mol%), P(n-Bu)Ad2 (7.5 mol%), K2CO2 (3 equiv.), and HOPiv (25 mol%) in DMA at 140 °C for 12 h has been clearly established as C-5 >> C-3, which allows the selective introduction of a new aryl group in that position. Now, if the reactivity of the C-3 position is so low, how can we arylate it? The trick is a transposition of the SEM group triggered by the use of a catalytic quantity of SEM-Cl (10%) in CH3CN at 95 °C for 24 h. The SEM shifts to the other nitrogen, mutating the numbering (and reactivity) of the ring so the former C-3 is now the C-5. A new arylation can be performed in the same way. The SEM group can be removed to yield the free pyrazole, but alternatively the alkylation of the first coupling product effects the same trick to yield in the end a 1-alkyl-3,4,5-arylatedpyrazole. Yields are excellent and examples with diverse phenyl rings and pyridines are described.
J. Am. Chem. Soc., 2009, 131 (8), pp 3042–3048. See: 10.1021/ja8096114