Palladium-Catalyzed, Ring-Forming Aromatic C–H Alkylations with Unactivated Alkyl Halides
Formation of carbocycles and saturated heterocycles through a palladium mediated intramolecular radical cyclization.
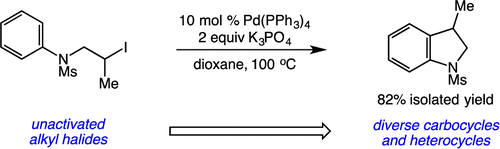
This is one of those papers that catch your attention because of its apparent simplicity, at least according to the graphical abstract (by the way, one of these days I will have to write something about that). The protocol developed by Alexanian et al. (University of North Carolina, NC, USA) is not fancy or an incredible breakthrough, but it works nicely making use of well known principles.
In short, they use Pd(PPh3)4 (yep, the old, dirty tetrakis) as catalyst and K3PO4 as base in dioxane to promote the cyclization of iodoalkylbenzenes through the formation of a radical. What makes this reaction interesting is that they do not activate the ortho position, which is where the cycle is formed. Just promoting the formation of the radical is enough to yield with good results both carbocycles (tetrahydronaphthalenes and indanes, 10 examples) and heterocycles (indolines, tetrahydroquinolines and tetrahydroisoquinolines among others, 18 examples). The method is compatible with boronic acid pinacol ester, ketone, alcohol and alkene, and obviously it has some issues with the regioselectivity when no symmetric iodoalkylbenzenes are used. Unfortunately the chirality in the halo-bearing carbon is destroyed during the radical formation (stereoablation, nice word). It would be interesting to know if you can use it also for oxygen-containing heterocycles…
J. Am. Chem. Soc. 2015, 137(11), pp 3731-3734.
See: 10.1021/jacs.5b01365