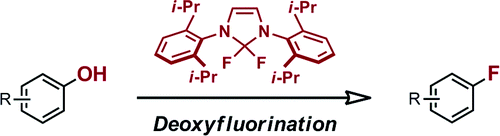
Deoxyfluorination of Phenols
Direct conversion phenols into fluorides with a new fluorination reagent.
Ritter (Harvard University, Mass., USA) has published a new method for direct fluorination of phenols making using of a new fluorinating agent, N,N’-1,3-bis(2,6-diisopropylphenyl)-2,2-difluoroimidazolidene. The preparation is described in the supporting info in a scale >100g and looks like a piece of cake. The protocol allows the direct fluorination of unprotected phenols; 30 examples are described with yields between 75 and 99%. Worst yield is 2-pyridone with a 50% (not surprising, honestly) and other heterocycles are described. Hmmm, I have in mind a couple of recent molecules where we could try this reagent…
J. Am. Chem. Soc. 2011, 133 (30), pp 11482–11484. See: 10.1021/ja2048072