Palladium- and Nickel-Catalyzed Cross-Couplings of Unsaturated Halides Bearing Relatively Acidic Protons with Organozinc Reagents
Zinc can do things that others just promise.
Medicinal chemists are quite familiar with different coupling reactions to introduce different groups. Suzuki, Sonogashira, Heck, Stille, Kumada, etc. There are plenty of other coupling reactions, but you will find that they are less known, which translates into less used when the need arises. The Negishi coupling is probably in this category, because many chemists have not ever tried one. One of the reasons for this lack of popularity is probably that there are no many organozinc reagents commercially available and usually you need to prepare them yourself. Moreover, the organozinc reagents are contemplated as unstable compounds with nothing to offer.
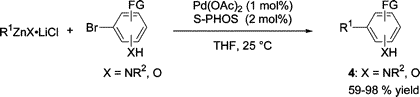
This new paper of Knochel (Munich, Germany) demonstrates not only that these reagents are quite friendly, but they can be used to do things which you can only dream of in other couplings. More specifically, you can use them with unprotected substrates bearing mildly acidic protons. In other words, normal chemists will protect any free hydroxy group before the coupling and everybody knows than benzylic groups can not be coupled.
The data published in the paper show that a wide range of organozinc reagents can be coupled with substrates containing free hydroxy and amino groups using Nickel or Palladium as catalysts. The organozinc reagents include benzyl, phenyl, pyridyl, octyl and other alyphatic structures. No problems are found using anilines, benzylic alcohols or phenols as partners. The use of this reaction can avoid tedious protection and deprotection steps. Sounds useful for preparing some libraries.
J. Org. Chem., 2008, 73 (21), pp. 8422–8436. See: 10.1021/jo8015852J