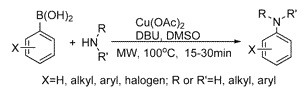
Microwave-Assisted Efficient Copper-Promoted N-Arylation of Amines with Arylboronic Acids
The Chan–Lam–Evans reaction is a recent addition to the arsenal of the modern chemist. Although it is a very interesting method for C–N bond generation, not many examples using microwaves are available.
Most chemists are familiar with the Ullmann reaction, a centenary method for amine arylation catalyzed by copper. For a long time, no new advances were made on this topic. On the last twenty years, a powerful, flexible method for the arylation of amines has emerged: the Buchwald–Hartwig amination. This method relies on the palladium-catalyzed coupling of a suitable halide (or triflate) with an amine. Protocols, examples, and modifications are available, and medicinal chemists use now this reaction on a daily basis.
The Chan–Lam–Evans coupling is a very interesting alternative to both the Ullmann and Buchwald–Hartwig couplings, involving the coupling of an arylboronic acid with an amine. This reaction provides not only an alternative method for the arylation of amines, but an alternative disconnection that can be made in case the halide is not available. In a few words, you can expand the preparation of amines with the big number of available commercial arylboronic acids.
Curiously, not many examples of the Chan–Lam–Evans reaction have been published (for an interesting review, see Angew. Chem. Int. Ed. 2003, 42, 5400) and the standard conditions can be enormously improved. A group of academic researchers in Shangai (China) has published a microwave-assisted modification of the reaction for the preparation of a wide range of arylamines in good yield. Although the reaction times are long for a microwave reaction (20–30 min), they are an improvement over ‘thermic’ times, which are measured in days. Examples include primary and secondary anilines and a few heterocyclic amines.
J. Am. Chem. Soc., 2007, 129 (51), pp. 15734–15735. See: 10.1021/cc8000053