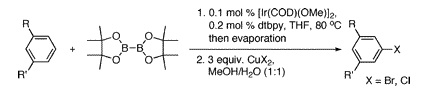
Meta-Halogenation of 1,3-Disubstituted Arenes via Iridium-Catalyzed Arene Borylation
Preparation of aryl halides is a key step in many syntheses, but the introduction of an halogen in meta to other substituents is not usually trivial. The key to that reaction is an iridium catalyst.
The halogenation of substituted aromatic compounds is the common way to access aryl halides. This reaction, an electrophilic aromatic substitution, usually yields the products resulting from ortho or para activation. Introduction of a halogen in meta position can therefore be troublesome and may involve the use of nitro groups, diazonium salts and tedious chemistry. Following their studies on C–H functionalization using iridium catalysts, the research group of Hartwig (Urbana, IL, USA) has published a short paper reporting the C–H borylation of 1,3-disubstituted arenes, followed by the use of a copper(II) halide. This methodology has been employed to prepare 1,3-substituted aryl bromides and chlorides. Both the catalyst and ligand are commercially available, and although the experimental details include a glovebox, more friendly Schlenk techniques can also be used.
J. Am. Chem. Soc., 2007, 129 (50), pp 15434–15435. See: 10.1021/ja076498n