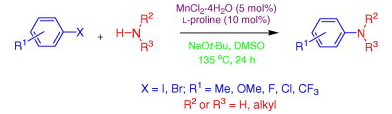Manganese-catalyzed cross-coupling reactions of aliphatic amines with aryl halides
Manganese-catalyzed introduction of aliphatic amines into aryl halides.
Sometimes I think that selecting papers for this newsletter is just a question of selecting new fancy couplings. It seems to be true for this issue, since but one, all papers selected are related with couplings. This one is related with the construction of C-N bonds, one of the most growing fields in the last years. The works by Buchwald, Hartwig and others allow the medicinal chemist to introduce a broad range of aminated compounds into aromatic rings by using Palladium chemistry. However, those methods have still some limitations, the most important being probably that most of the work is focused on the arylation of amines, while efficient alkylation protocols are still underdeveloped. Our own company has contributed to this field with a couple of papers based on the use of the Chan-Lam reaction, a Copper-based protocol; the first one is aimed at the monomethylation of aromatic amines, while the second expands the initial method to allow the introduction of other alkyl chains.
Another point is the strategy employed in the construction of the C-N bond in terms of starting materials. The Buchwald-Hartwig approach involves Ar-X + Ar-NH2 with Pd; the Ullmann reaction Ar-X + Ar-NH2 with Cu; the Chan-Lam approach involves Ar-NH2 + R-B(OH)2 (where R can be now aryl or alkyl) with Cu; and so on.
The paper we present here by Teo et al. (Nanyang Technological University, Singapore) is focused not on the formation of Aryl-N-Aryl bonds, but Aryl-N-Alkyl bonds using the strategy Ar-X + R-NH2, where R is exclusively an aliphatic group and the metal involved is Manganese. In a typical reaction the aryl halide and the amine are mixed with MnCl2·4H2O (5 mol%), L-proline (10 mol%) and t-BuONa (2.0 equiv) in DMSO at 135 °C for 24 h. A nice mix of substrates is presented, with yields ranging between good and very good (80% is the best yield obtained). Since the solvent is DMSO this reaction can be probably nicely done under microwave heating.
Tetrahedron Lett. 2010, 51 (30), pp 3910-3912. See: 10.1016/j.tetlet.2010.05.098