An Alkyne Hydroacylation Route to Highly Substituted Furans
Rhodium catalyzed synthesis of linked-furanes.
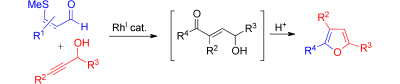
I know that furanes are not popular among the med chem community (structural alerts and so), but we have at least a marketed drug with a furane (ranitidine), so these poor small cycles deserve an opportunity. This paper by Willis et al. (University of Oxford, UK) shows a method for the preparation of di- and trisubstituted furanes starting from small acyclic precursors. The method makes use of a Rhodium catalyst (Rh(nbd)2]BF4, aka Bis(norbornadiene)rhodium(I)tetrafluoroborate; don’t worry, is commercial). They report 15 examples of furanes which bear a linker with a thioether moiety in the end (this is looking uglier by moments, uh?). Changing the starting materials, you can prepare also 2,5-disubstituted pyrroles, thiophenes and 2,6-disubstituted pyrazines, though only one example of each is provided. Probably the authors will write down another paper with those. So next time you look for furanes with a linker, think about this method.
By the way, look at the graphical abstract and tell me why, if they use red and blue in the starting materials and the products, they do not color also the intermediate?
Angew. Chem. Int. Ed. 2011, 50, pp 10657 –10660. See: 10.1002/anie.201105795