Rapid and easy access to indoles via microwave-assisted Hemetsberger–Knittel synthesis
A straighforward synthesis of indoles using microwave technology.
The synthesis of indole derivatives has been a major topic in organic and medicinal chemistry over the past several decades. One of the myths of organic synthetic chemistry, Emil Fischer, devised a synthesis that has been one of the most used methods for the preparation of indoles. Many other methods are available, each with its successes and shortcomings, but still new methods are published each year.
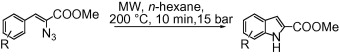
This paper by Laufer (Tuebingen, Germany) is one of those striking examples of how an old reaction is vastly improved using microwave technology. The Hemetsberger–Knittel synthesis involves the condensation of an arylaldehyde and an azidoacetate to provide a-azidocinnamates, which upon heating give indoles; hiwever, as usual, the harsh conditions render the method less than ideal. After a short screening, conditions comprising 200 W, 200 °C, and 10 min of microwave irradiation were set as the standard method to synthesize different indole-2-carboxylates from aldehydes.
Tetrahedron Lett., 2009, 50, pp 1708–1709. See: 10.1016/j.tetlet.2009.01.129