Microwave-Assisted Synthesis of Weinreb and MAP Aryl Amides via Pd-Catalyzed Heck Aminocarbonylation Using Mo(CO)6 or W(CO)6
Preparation of Weinreb amides from scratch.
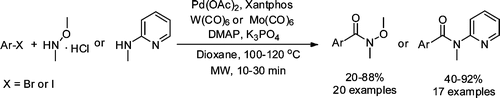
Weinreb amides (N-methoxy-N-methylamides) have many important synthetic uses and therefore new approaches are always welcomed. It is not the first time we have included in our Top Five a paper on the preparation of Weinreb amides. In fact, so far we have selected papers describing its preparation by coupling with CO and N,O-dimethylhydroxylamine, improved methods of preparation from the carboxylic acids and a coupling reaction which makes use of boronic acids instead of aryl halides.
This paper by Odell (Uppsala, Sweden) falls in the couplings cathegory. It is in fact a nice refinement of the work by Buchwald, where the CO gas has been replaced with a more tractable source: a CO complex of Molibdene or Tungsten. Not only that, but the method includes now the use of microwave heating, so the reaction time is the range of minutes, specially suited for drug discovery projects. In a typical reaction, the aryl halide and N,O-dimethylhydroxylamine are mixed with Pd(OAc2) (5 mol%), Xantphos (10 mol%), Mo(CO)6 (33 mol%), DMAP (200 mol%) and K3PO4 in dioxane at 110 °C. The mixture is subjected to microwave irradiation for 10 minutes. The yields are generally good. A similar protocol can be followed for the preparation of MAP amides.