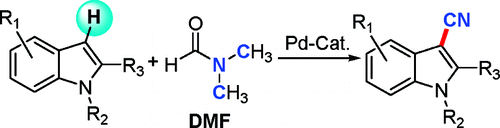
Direct Transformation of N,N-Dimethylformamide to -CN: Pd-Catalyzed Cyanation of Heteroarenes via C-H Functionalization
Cyanation of protected, C-2 substituted indoles, without using a cyanide source, only DMF.
This work by Jiao (Shanghai, China) describes an interesting cyanation method for N-protected, C-2 substituted indoles using no cyanide source, but DMF. The reaction protocol uses a lot of things (Pd, Cu, Fe), but in the end you will get your indole with a CN group in the C-3. 28 examples are described, including two benzofuranes. Yields are generally acceptable, though nothing to write home about (40-80%). A backdraw is that the main byproduct is the formylated compound (or an advantage, depending what you are going to do), but selectivies are usually excellent. It is a nice paper, with a lot of science and effort behind devoted to elucidate the mechanism. I would love to see more chinese papers so good and sound as this one.
J. Am. Chem. Soc. 2011, 133 (32), pp 12374–12377. See: 10.1021/ja204063z