A General and Efficient Catalyst for Palladium-Catalyzed C-O Coupling Reactions of Aryl Halides with Primary Alcohols
Introduction of primary alcohols using coupling reactions.
Let’s be clear about this: some groups are introduced and others, you take them as they come. When you think about this curious statement, you will realize that aryl ethers are among the second group; aromatic ethers are so common that we usually look for commercial substrates with the proper substitution pattern, but if we need nitrogen, halogens or others, we start thinking about nitrations, brominations, and so on.
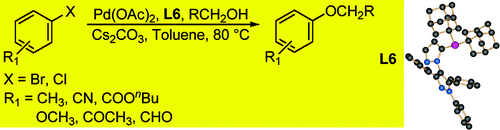
Well, methods for the introduction of C-O bonds (besides the old Williamson reaction) exist. And the paper by Beller et al. (Rostock, Germany) is a good example. The method relies in the use of two bulky ligands, Singer’s di-tert-butyl-substituted Bippyphos and the even more bulky analog where the two tert-butyl groups are replaced by adamantyl groups.
In a typical reaction the aryl halide (chloride or bromide) is mixed with the ligand (2 mol%), the primary alcohol (3 equiv.), Cs2CO3 (1.5 equiv) in toluene at 80 °C for 3-6 h. Most of the examples in the paper are run with n-BuOH, but other more complicated alcohols can be used. In fact the method discriminates between primary (reactive) and secondary or tertiary hydroxy groups (unreactive), so there is no need to use protecting groups when using diols, for example. Very nicely the authors have included also a table with results obtained from heterocyclic halides (mostly pyridines), in excellent yields. A reaction to be considered next time you think about changing the linker.
J. Am. Chem. Soc., 2010, 132 (33), pp 11592–11598. See: 10.1021/ja103248d