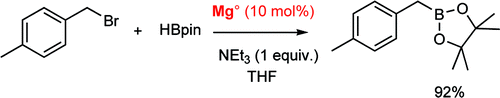An Opportunity for Mg-Catalyzed Grignard-Type Reactions: Direct Coupling of Benzylic Halides with Pinacolborane with 10 mol % of Magnesium
Preparation of benzylic boronates using the Grignard reaction.
Breaking news! Heck, Negishi and Suzuki win the Nobel Prize!
I think everybody is aware of this well deserved achievement, so I suppose talking about boronates and how useful they are is not necessary.
This paper by Duñach et al. (CNRS, France) clearly shows how far the chemistry of these products has gone. Classic preparation of boronates using magnesium involves generating the Grignard reagent and then allowing it to react with a trialkoxyborane, generally at low temperature. But it seems until now nobody had made a very interesting observation: the borylation of several aryl and benzyl halides with pinacolborane sometimes do not consume all the Magnesium. This observation prompted the authors to try the reaction with a catalytic amount of Magnesium (10%). To their surprise, the reaction works smoothly to give the boronate in good yields, at least in most cases. The protocol is easy: just add the benzyl bromide, then triethylamine and finally pinacolborane to Magnesium turnings (10 mol%) in distilled THF and stir at reflux for 15 h. The benzylboronic pinacol esters are obtained in yields from good to excellent. Things are more complicated when benzyl chlorides or secondary benzyl halides are used as starting materials, but even then acceptable yields are obtained. Not a bad idea to avoid using a lot of those nasty Magnesium turnings.
J. Am. Chem. Soc., 2010, 132 (34), pp 11825–11827. See: 10.1021/ja1052973