Copper-Catalyzed C(sp3)–C(sp3) Bond Formation Using a Hypervalent Iodine Reagent: An Efficient Allylic Trifluoromethylation
Introduction of the trifluoromethyl moiety in allylic positions.
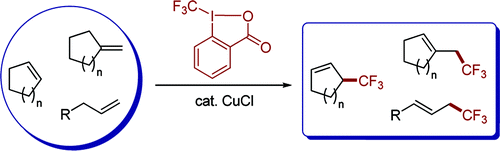
This paper by Wang et al. (Beijing National Laboratory of Molecular Sciences, Beijing, China) looks like a nice addition to the medicinal chemist weaponry. They report the use of a hypervalent iodine reagent to effect the trifluoromethylation of allylic compounds. In fact, what they really do is TO introduce a CF3 into the terminal position of the alkene, which in turns migrates internally so the new moiety is now in the allylic position. So if you have an allyl group hanging, you will end with a 1,1,1-trifluorobut-en-1-yl chain instead. It works also on cyclic and exocyclic alkenes. About 15 examples are reported, with good yields. I foresee some interesting uses for reluctant metabolites.
J. Am. Chem. Soc., Article ASAP. See: 10.1021/ja207775a