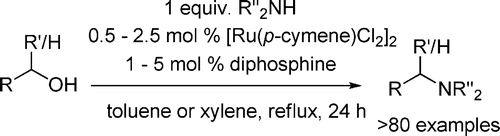Ruthenium-Catalyzed N-Alkylation of Amines and Sulfonamides Using Borrowing Hydrogen Methodology
A powerful alternative to reductive amination using alcohols and a ruthenium catalyst.
Reductive amination is probably one of the most used reactions in medicinal chemistry and the method of choice for the preparation of substituted amines. However, the reaction has a few shortcomings and limitations, being the most obvious the use of a reducing agent that sometimes is not compatible with other groups present in the substrates. There is a more intrinsic, almost philosophical limitation: carbonyl compounds are used. Many times these reagents are available, but otherwise they have to be prepared by oxidation of the corresponding alcohols, which always adds one additional step.
The paper from Williams et al. (University of Bath, UK) gives now a new way into amines using alcohols. Similar reactions have been reported using other ruthenium and iridium catalysts. The new method reported relies on the use of a ruthenium catalyst in the form of [Ru(p-cymene)Cl2]2 in the presence of a phosphine ligand. Both ddpf and DPEphos give good results. A typical example is the benzylation of a series of amines using benzyl alcohol, 1.25 mol% of [Ru(p-cymene)Cl2]2 and 2.5 mol% of the ligand in toluene at reflux for 24 h. In this particular case, DPEphos gives consistently better results than dppf, although the yields are excellent anyway.
The authors have explored thoroughly the scope of the reaction, applying it to other alcohols, both aliphatic and aromatic, and the alkylation of dimethylamine or dimethylammonium acetate, among others. The reaction is flexible and very powerful: for example, cyclic amines can be constructed using as partners 1,4-butanediol, 1,5-pentanediol or 1,6-heptanediol. Moreover, the alkylation of sulfonamides can also be performed. The reaction has a limitation, however, regarding the use of chiral alcohols, whose chirality is destroyed in the reaction. On the other hand, chiral amines retain the chirality. The method is simple and relatively inexpensive and both the catalyst and ligands are commercial. Sounds good, right?
J. Am. Chem. Soc., 2009, 131 (5), pp. 1766–1774. See: 10.1021/ja807323a