Reactivity of a Recombinant Esterase from Thermus thermophilus HB27 in Aqueous and Organic Media
Biocatalysis has the potential to impact greatly the chemical industry, by reducing the environmental impact and increasing economic benefits. Through the use of enzymes, the desired products may be obtained in an environmentally friendly manner, requiring fewer chemicals and energy, and producing fewer undesirable by-products than by traditional catalytic processes.
The main drawback associated with the use of many enzymes is their limited stability under the conditions employed in most industrial bio-manufacturing processes. In this context, enzymes obtained from extreme thermophiles can offer not only thermal stability, but stability against a number of denaturing agents, including organic solvents.
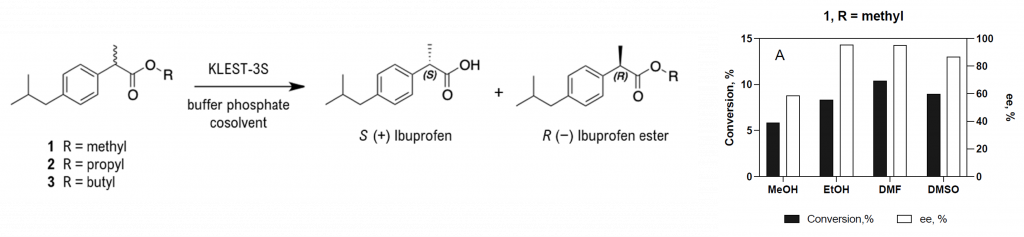
The aim of this work was to gain insight on the catalytic properties of esterase KLEST-3S, particularly in organic media and on substrates of commercial interest. This recombinant esterase was found to display high thermal stability, being active in the presence of 10% (v/v) organic solvents and 1% (w/v) detergents. KLEST-3S hydrolyzed triglycerides of various acyl chains, which is a rare characteristic among carboxylic ester hydrolases from extreme thermophiles, with maximum activity on tributyrin. The esterase provided high yields for the acetylation of alcohols in organic media. In addition, recombinant KLEST-3S was able to catalyze the stereoselective hydrolysis of racemic ibuprofen methyl ester with DMF or DMSO as organic solvent yielding the medically relevant S-enantiomer (87% ee in DMF).
You can read the full paper here: Microorganisms 2022, 10, 915. DOI: 10.3390/microorganisms10050915 (Open Access)
The present work was carried out within the project HOTDROPS, which ran from 2013 to 2017 with funding from the European Union through the Seventh Framework Programme – Industry-Academia Partnerships and Pathways – Marie Curie Actions (FP7-IAPP-Marie Curie).


