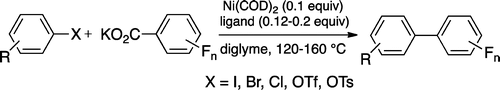Nickel-Catalyzed Decarboxylative Cross-Coupling of Perfluorobenzoates with Aryl Halides and Sulfonates
Conditions to carry out a Suzuki-like coupling with perfluorinated benzoates.
I have said this before: today we can couple everything. The days when you needed a iodide or bromide as starting material are gone. They ended a long time ago indeed. We can couple iodides, bromides, chlorides, fluorides, tosylates, triflates, phosphonates, etc… and the partners can be a wide range of compounds bearing boron, tin, magnesium and others. But not only those. Carboxylates can also be used as partners, as in this particular case.
Honestly, I had my doubts when I selected this paper: Having to introduce a perfluorinated phenyl ring is not something that do you every day. I remember a presentation during a SEQT event in 2012, when a researcher showed very good results with some analogs, including one with a perfluorinated phenyl ring. Since no more examples with phenyl rings where included, the expected question was ‘The use of that perfluorinated ring is very important for activity?’. And the answer was no. The reagent was at hand, so they used it. But sometimes you really have to put those fluorine-rich rings into the molecule. And usually is not an easy task.
Kalyani et al. (St. Olaf College, MN, USA) have developed a new powerful protocol to carry out that reaction, and it is so versatile that they can use chlorides, bromides, iodides, tosylates and triflates, with potassium perfluorobenzoates as partners. The decarboxylative coupling is carried out using Ni(COD)2 as catalyst in diglyme, heating at 120-160 °C. Plenty of examples with both the Ar-X and three different fluorobenzoates. There is, however, a disturbing lack of haloheterocycles.
Org. Lett. 2015, Article ASAP.
See: 10.1021/acs.orglett.5b00241