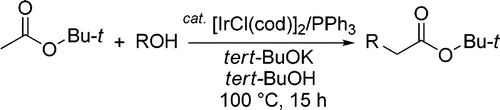Iridium-Catalyzed a-Alkylation of Acetates with Primary Alcohols and Diols
A method for the alkylation of acetates using Iridium.
Aldolic condensation is one of the oldest reactions around. Well known, widely used and powerful, it has figured among the number one methods for creating C-C bonds for a long time. But times change and we have greener alternatives for old methods. The Iridium catalyzed alkylation of acetates is one of those green chemistry methods, the green part being the use of alcoxide bases, not the usual lithium salts, high temperatures and strikingly, alcohols as partners instead ketones or aldehydes.
The method published by Ishii (Kansai University, Osaka, Japan) is quite simple: t-butyl acetate and an alcohol are allowed to react in the presence of an Iridium catalyst (5 mol%), PPh3 (15 mol%) and t-BuOK (20 mol%) in t-BuOH at 100 °C for 15 h. The best Iridium catalyst for the transformation is [IrCl(cod)]2 with yields ranging from 64% to 90%. The method allows aliphatic and benzylic alcohols to be used, but only primary alcohols. Looks like a good method for preparing phenylacetic acids.
J. Am. Chem. Soc., 2010, 132 (8), pp 2536–2537. See: 10.1021/ja9106989