Scope of Aminomethylations via Suzuki–Miyaura Cross-Coupling of Organotrifluoroborates
Once again, the development of an organometallic cross-coupling reaction increases the array of available methods to prepare amines. In this case, an alternative to reductive amination.
Most organic chemists have probably carried out at least one reductive amination during their career. It is the number one option when preparing amines, since it avoids the usual problems encountered when amines are subjected to nucleophilic substitution. Many high-order amines can be prepared from a carbonyl group and simpler amines, and the reaction allows different disconnections to be done in order to get the desired product. The paper of Abdel-Magid et al. (J. Org. Chem. 1996, 61, pp. 3849–3862) is a classical reference.
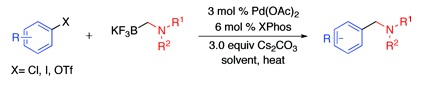
Now, a work by Molander (Philadelphia, PA, USA) goes a little further. The usual sequence for the introduction of a dialkylaminomethyl group on an (hetero)aromatic ring involves the use of an aldehyde, which in turn can be prepared from a bromide derivative by metallation and trapping with DMF, with an amine under reductive amination conditions. This new method consists in the coupling of N,N-dialkylaminomethyltrifluoroborates with an appropriate substrate (chloride, bromide, iodide, or triflate) in the presence of a common palladium catalyst, XPhos and cesium carbonate. The N,N-dialkylaminomethyltrifluoroborates can be easily accessed from potassium bromomethyltrifluoroborate and commercial amines in gram quantities. Since no reduction agents are used, this method is compatible with other groups sensitive to common reductors.
J. Org. Chem. ASAP. See: 10.1021/jo800183q