Microwaves are one of the most useful tools for medicinal chemists. Reactions that typically required hours can now be carried out in minutes using a microwave reactor. We have developed a new method for the microwave-assisted aza-Cope rearrangement of N-allylanilines.
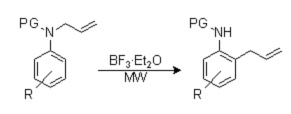
As part of our own R&D projects, we develop new synthetic methods for known and new scaffolds. Ortho-allylanilines are interesting substrates for the preparation of different bicyclic nitrogen-containing heterocycles through cyclization of the nitrogen onto the double bond. The usual preparation method for these products is the rearrangement of aromatic N-allylamines, but the harsh reaction conditions limit the use of the aza-Cope rearrangement. Moreover, the intermediate ortho-allylanilines are not isolated and its preparation is poorly described in the literature.
The requirements of our project and the absence of a really efficient method prompted us to undertake a comprehensive study of the use of microwave-assisted reaction conditions for this transformation. We have found that protected N-allylanilines with different substitutions on the aryl ring can be transformed under microwave irradiation in the presence of BF3·Et2O within 1–2 min at 170 °C. This work has been accepted for publication in the journal Tetrahedron Letters.